From urgency to action: how DemcAir delivered fast.
We developed the DemcAir during 2020 for the Dutch Government with the objective to support Covid-19 patients on ICU’s. We offer a solution for a short time to market introduction of your own branded ICU ventilator, by licensing of the DemcAir. The DemcAir can be adapted to your own specifications and wishes in order to make an indigenous and unique ICU ventilator.
highlights
- Licensing and customization
- Shortest time to market
- (technical) Support regarding CE and FDA certification and preparation of local production
robust solution for respiratory challenges.
The DemcAir ventilator is designed for rapid deployment and customization solutions. The systems features a turbine-driven architecture with an intuitive user interface, ready to license for local sourcing and assembly. The license includes all detailed mechanical, electrical and software documentation including GUI source code and CAD files along with fully verified design and assembly instructions. This CE-ready solution, supported by pre-compliance testing, can be customized to meet your specific requirements. The DemcAir can be evaluated at your own site and experience Macawi’s innovative respiratory technology firsthand. Our engineering team is available online via video connection to provide technical support throughout the process.
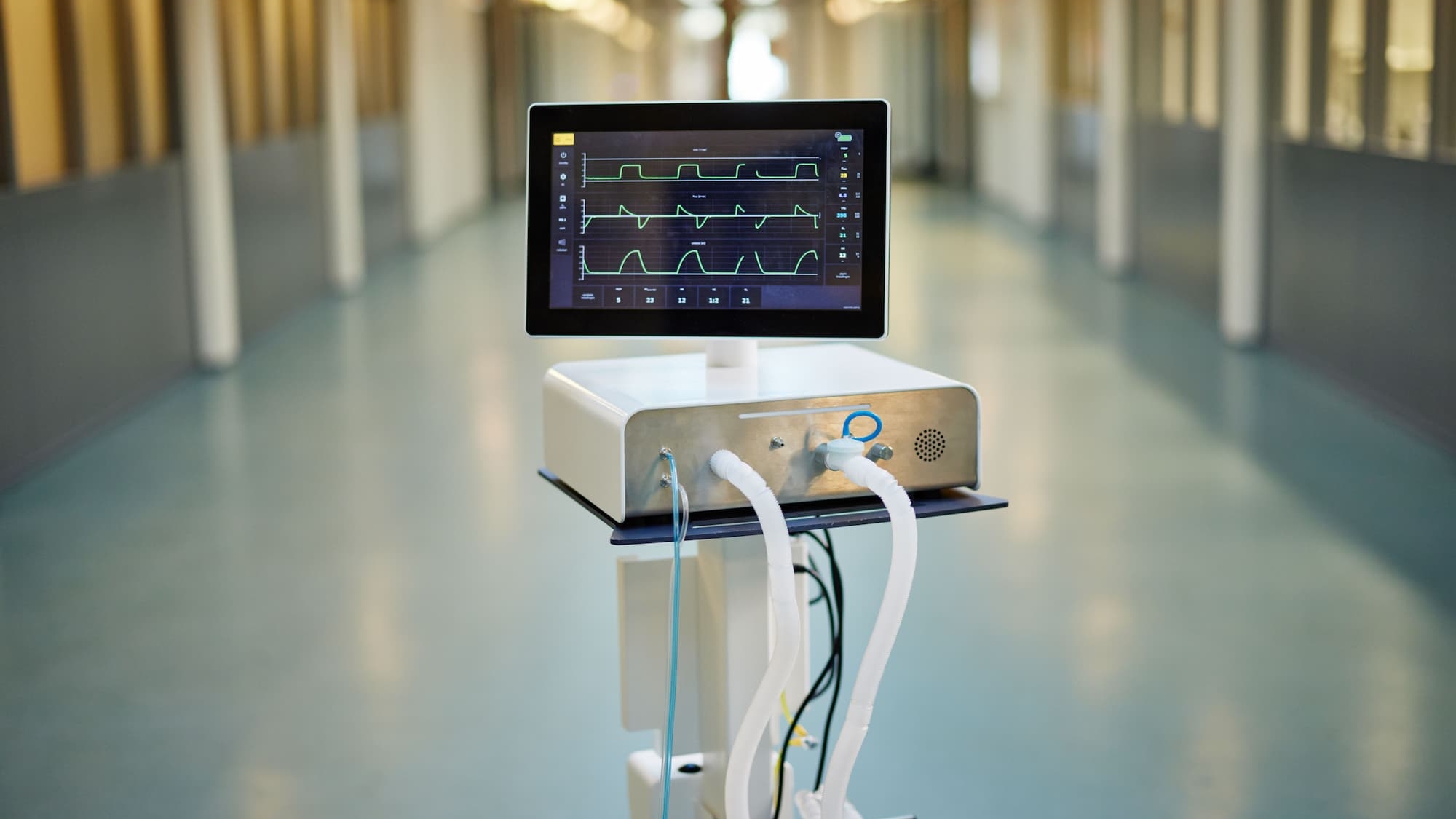
fully customizable.
ready to explore the possibilities?
Interested in exploring the possibilities for your ventilator development project? Or would you like to learn more about the DemcAir and how it can accelerate your time to market? We’d love to get in touch and discuss your ideas.